Muva nje, i-Shanghai Chuangkun Biotechnology Co., Ltd. ithole isitifiketi sesibili sokubhalisa se-Indonesia FDA sekhithi yokutholwa kwe-TB/NTM Nucleic Acid(lyophilized), sinyathelwe (12+3)ikhithi ye-PCR yohlobo lwe-HPV enyangeni eyodwa edlule.eyamemezela ukuthi imikhiqizo ye-Chuangkun Biotech igunyazwe yi-Indonesia FDA, yenza ngcono imikhiqizo ezithuthukisayo, futhi inikeze nokusekelwa okuqinile kwe-Chuangkun Biotech ukuze iqhubeke nokwandisa imakethe yamazwe ngamazwe.
Ngokombiko we-World Health Organization's Global TB Report 2022, balinganiselwa ezigidini ezingu-10.6 abantu abazophathwa i-TB ngo-2021. Izinga elandayo ngo-4.5% lalingaphezu konyaka ka-2020. abantu abaphila ne-HIV).Ngokwendawo, izigameko eziningi ze-TB ngo-2021 zazisezifundeni ze-WHO eNingizimu-Mpumalanga ye-Asia (45%), e-Afrika (23%) naseWestern Pacific (18%), zinamasheya amancane eMpumalanga Mediterranean (8.1%), emazweni aseMelika. (2.9%) kanye neYurophu (2.2%).Uma kucatshangelwa ubhubhane lwe-COVID-19, ukuvimbela nokulawula isifo sofuba kuya ngokuya kuhlukumeza oHlelweni Lokuqedwa Kwesifo Sofuba lwe-WHO.
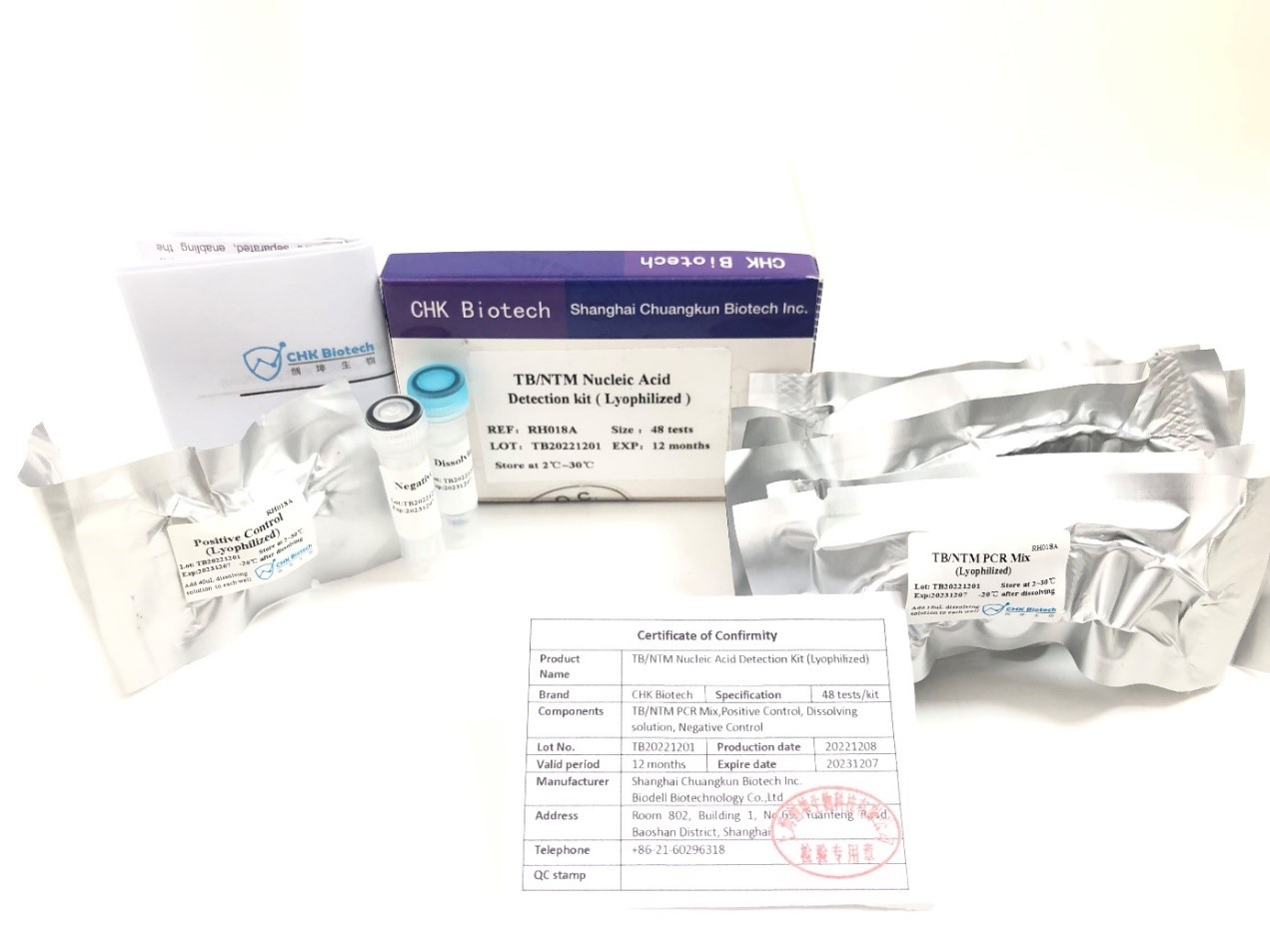
Ikhithi yokuthola i-TB/NTM Nucleic Acid evela ku-CHUANGKUN Biotech idalwe ngenqubo ye-lyophilization .Le nqubo izoxazulula ububi besikhathi eside bezinto zokuhamba zekhithi yokutholwa ye-PCR evamile, futhi iletha ukunemba okuhle kakhulu kanye nemiphumela ecacile kakhulu ngaphezu kokusebenziseka kwethu.
I-Shanghai Chuangkun Biotechnology Co., Ltd. ithole isitifiketi sesibili sokubhalisa se-Indonesia FDA sekhithi yokuthola i-TB/NTM Nucleic Acid, esibonisa ukuqashelwa yi-Indonesia FDA.I-Chuangkun Biotech izozinikela ekubambeni iqhaza ekuvimbeleni nasekulawuleni isifo sofuba sesifunda.
Isikhathi sokuthumela: Jun-08-2023

 中文
中文